Digital Clinical Trials Market Growth, Size, Share and Projected to Reach USD 22.50 Billion by 2031
Digital Clinical Trials Market Set for Strong Growth Driven by Rising Adoption of Telehealth and Technological Advancements
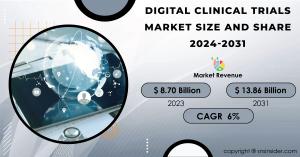
AUSTIN, TEXAS, UNITED STATES, May 15, 2024 /EINPresswire.com/ -- The Digital Clinical Trials Market, valued at USD 8.70 Billion in 2023, is anticipated to reach a valuation of USD 13.86 Billion by 2031. This translates to a compound annual growth rate (CAGR) of 6% throughout the forecast period from 2024 to 2031.List of Digital Clinical Trials Companies Profiled in Report:
• ICON, plc
• PPD
• Parexel International Corporation
• Human first
• IQVIA
• Covance
• IBM
• PRA Health Sciences
• Deloitte
• Data Management 365
• LEO Innovation Lab
• Medidata
• Oracle
• CRF Health
• Clinical Ink
• Medable, Inc.
• Signant Health
• Halo Health Systems
• Croprime
Download Free Sample Report of Digital Clinical Trials Market @ https://www.snsinsider.com/sample-request/2690
Rise in R&D activities, healthcare digitization, and telehealth adoption
Increased investments in research and development, coupled with the growing digitization of healthcare and the widespread adoption of telehealth solutions, are creating a fertile ground for the digital clinical trials market.
Technological advancements
Advancements in mobile & wearables, artificial intelligence (AI), cloud technology, and associated platforms are enabling the collection of frequent, accurate, and multidimensional data throughout clinical trials. This fosters innovative trial designs that enhance patient recruitment and retention, improve overall patient experience, and establish novel endpoints in clinical studies.
In July 2023, Signant Health completed the acquisition of DSG, strategically expanding its eClinical solution suite for both traditional and decentralized clinical trials. This acquisition facilitated the development of a comprehensive trial ecosystem equipped with advanced software, analytics, and logistics solutions, enabling seamless study conduct and data generation across various modalities. This ultimately contributes to the goal of fully digitalizing clinical trials.
In June 2023, Medable Inc. introduced a comprehensive toolkit specifically designed for Institutional Review Boards (IRBs) and Ethics Committees (ECs). This toolkit streamlines the process of establishing standardized ethics review procedures for decentralized clinical trials (DCTs). The implementation of this toolkit simplifies, expedites, and enhances the IRB/EC process, fostering greater efficiency and a more patient-centric approach in the execution of DCTs.
Patient-centric approach
A new strategy called patient-centricity is emerging to ensure efficient clinical trials based on new perspectives. This approach involves aligning business development strategies with the design of new apps and wearables that enhance data collection and improve direct communication between patients and sponsors. These apps and wearables, directly connected to patients' phones, allow for real-time data collection based on daily activities, enriching the clinical trial process.
Virtual methods for decentralized trials
Virtual methods offer a novel approach to clinical research, allowing participants to take part in trials from their homes, ensuring research continuity even when in-person visits are not feasible. Virtual visits and remote patient monitoring provide participants with greater flexibility and peace of mind, minimizing unnecessary travel and potential risks. This approach also enables sponsors to include a broader population in studies, enhancing recruitment, engagement, and retention rates. Additionally, continuous real-time data collection through digital health technologies streamlines the research process. Ultimately, virtual connectivity, monitoring, and management significantly reduce effort, time commitment, and burden on participants, clinical research coordinators (CRCs), and investigators.
Have Any Query? Ask Our Experts @ https://www.snsinsider.com/enquiry/2690
Segment Analysis
Phase II: The Phase II segment dominated the virtual clinical trials market in 2023, accounting for the largest revenue share of over 32%. This dominance can be attributed to the widespread adoption of digital clinical trial (DCT) tools and platforms in Phase II and Phase I clinical trial procedures for patient participation. Virtual clinical trials are particularly beneficial in Phase II as they offer significant value to the biopharmaceutical and pharmaceutical industry by saving time-sensitive patient data, reducing delays in approvals, and minimizing site payments. Traditional Phase II trials are often the most expensive due to the high costs associated with recruiting participants and conducting in-person visits at central clinical facilities. Virtual Phase II trials, conducted remotely via the internet, streamline the process from recruitment and screening to data collection, eliminating travel burdens for participants and significantly reducing trial costs.
Phase III: The Phase III segment is projected to register the fastest CAGR over the forecast period. Digital advancements encompassing Big Data, AI, cloud computing, robotics, and social media are creating opportunities to revolutionize clinical trial processes. Some pharmaceutical companies are conducting virtual trials alongside traditional on-site Phase III and Phase IV trials to gather additional data from diverse patient populations across the globe. Virtual clinical trials in Phase III offer the potential to automate data collection, enhance patient engagement and retention, and facilitate real-time data access for trial investigators through monitoring devices. These combined benefits are anticipated to drive lucrative growth opportunities within this segment in the near future.
North America Is Expected To Maintain Its Dominance In The Global Digital Clinical Trials Market Throughout The Forecast Period
North America has a well-established healthcare infrastructure with high investments in R&D activities, fostering the adoption of innovative technologies in clinical research. Supportive government regulations and initiatives promoting the use of digital technologies in clinical trials further propel market growth in this region.
The Asia Pacific Region Is Anticipated To Witness The Fastest Growth In The Digital Clinical Trials Market Over The Coming Years
The increasing prevalence of chronic diseases such as cardiovascular diseases and cancer in the region is creating a demand for efficient and cost-effective clinical trials to develop new treatment options. The expanding geriatric population in this region presents a significant target audience for clinical trials, and virtual trials offer a convenient and accessible way for this demographic to participate in research.
Governments in the Asia Pacific region are increasingly recognizing the potential of digital clinical trials and are implementing policies to facilitate their adoption.
Key Takeaways for the Digital Clinical Trials Market
• Gain insights into the current market size of the digital clinical trials market and its projected growth trajectory over the forecast period.
• Understand the performance of different segments within the market, including phase, technology, therapeutic area, and region.
• Get insights into the factors that are propelling the growth of the digital clinical trials market and the potential challenges that may hinder its progress.
• Gain valuable information about the key players operating in the market, their product offerings, and their strategic initiatives.
• Stay ahead of the curve by understanding the latest trends and developments that are shaping the future of the digital clinical trials market.
Purchase Digital Clinical Trials Market Report @ https://www.snsinsider.com/checkout/2690
Akash Anand
SNS Insider Pvt. Ltd
+1 415-230-0044
email us here
Visit us on social media:
Facebook
Twitter
LinkedIn
Instagram
YouTube
Distribution channels: Healthcare & Pharmaceuticals Industry
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release