Advancing Gene-Modified, Inactivated HIV-1 Vaccines Through Clinical Studies: Novel Approaches Merit Specialized Manufacturing CapabilitiesAdvancing Gene-Modified, Inactivated HIV-1 Vaccines Through Clinical Studies: Novel Approaches Merit Specialized Manufacturing Capabilities
The world has reached another mile marker on the long and arduous road to eliminating human immunodeficiency virus (HIV) as a public-health threat. In June 2021, United Nations (UN) member states adopted strategic commitments for maximizing access to HIV services, with 2025 serving as a checkpoint on the path to ending the acquired immunodeficiency syndrome (AIDS) pandemic by 2030. The Joint UN Programme on HIV/AIDS (UNAIDS) called upon countries to boost implementation of evidence-based measures for decreasing HIV transmission and increasing availability of services, especially for at-risk populations and in regions with significant gaps in healthcare capacity. Building upon successes and challenges from a similar initiative executed between 2014 and 2020, UNAIDS set ambitious goals for countries to achieve going into 2025 (1):
∙ Reduce new HIV infections to <370,000
∙ Ensure that 95% of people at risk of infection within all epidemiologically relevant populations and geographies can access — and ultimately use — “appropriate, prioritized, person-centred, and effective combination prevention options”
∙ Reduce new HIV infections among adolescent girls and young women to <50,000
∙ Ensure availability of preexposure prophylactic (PrEP) medication for 10 million people at risk of infection as well as access to postexposure prophylactic (PEP) treatment for recently exposed individuals
∙ Provide appropriate and prioritized combination prevention options to 95% of at-risk people within humanitarian settings.
To gauge progress toward those goals, UNAIDS designed additional metrics, often called the 95–95–95 targets. By 2025, countries would strive for 95% of HIV-positive individuals to know their infection status, 95% of diagnosed individuals to receive antiretroviral therapy (ART), and 95% of treated individuals to exhibit suppressed viral loads (1).
Although health agencies continue to collect data for the 2025 checkpoint, the world is unlikely to have met the 95–95–95 targets — but not for lack of trying. For instance, Botswana, Eswatini, Malawi, Rwanda, and Tanzania all had reached those goals by the end of 2021, and several other African nations were on pace to do so by 2025 (2, 3). However, progress has been uneven globally (2, 3). Myriad, interconnected factors account for such disparity, including differences in wealth distribution, public-health infrastructure, and political will — not to mention varying levels of stigma against preventive measures and at-risk populations. Moreover, ART remains expensive despite increasing availability of generic products and efforts by international aid organizations to provide low-income countries with drugs at significantly reduced prices (4). Consider that, in 2023, an estimated 39.9 million people around the world were living with HIV, 1.3 million of whom represented new infections (5). That same year, 630,000 people died from AIDS-related illnesses (5).
Arguably, low-cost preventive vaccines still represent the best option for ending the long HIV/AIDS health emergency. As Eric Le Forestier (general manager of Naobios) told me at the end of 2024, “There are already a few therapies that work fairly well in removing virus from the bloodstream of an infected person. So maybe there is less interest for a vaccine, but that may be true for richer countries, where people can afford those treatments.” With pricing being such an acute factor, he added, “There is still a big interest for vaccines for developing countries because, in theory, vaccines would be less expensive.” Experts in public health and organizations such as the US National Institute of Allergy and Infectious Diseases (NIAID) concur, also asserting that vaccines would provide “durable” means for HIV mitigation (2, 6, 7).
Of course, HIV-vaccine research and development (R&D) has vacillated between high hopes and significant setbacks going back to the 1987 VaxSyn clinical trial of a candidate based on recombinant viral-envelope (env) glycoprotein 160 (gp160) (8). Several product candidates have cleared early safety studies only to underperform in later phases, insufficiently conferring immunity to vaccinated subjects. Recently terminated trials include NIAID’s phase 2b/3a Uhambo study of a vaccine based on a canarypox vector and purified gp120 (discontinued in 2020) and Janssen’s/Johnson & Johnson’s phase-3 Imbokodo and Mosaico trials for candidates based on adenovirus 25 (Ad25) vectors and purified gp140 (discontinued in 2021 and 2023, respectively) (9–11).
But even terminated clinical trials are shedding light on virology and correlates of immunity (12). Moreover, continuing advances in genetic engineering are illuminating R&D pathways that would have seemed impossible even a decade or two ago. Representing one such approach is an HIV-1 vaccine candidate developed by Sumagen Canada Inc., a wholly owned subsidiary of Korea-based Creo SG Co., Ltd. According to Eunsil Choi (director of drug development at Sumagen Canada Inc./Creo SG Co., Ltd., hereafter called “Creo SG”), the SAV001 candidate is “a killed, whole-virus vaccine containing a genetically modified HIV-1 that has been inactivated both chemically and by gamma irradiation.” The gene-modification process is designed to minimize viral infectivity, surmounting historical concerns about the safety of using inactivated or attenuated HIV for vaccination. However, production of SAV001 “involves cultivating and handling large amounts of live HIV.” As with other agents that can cause serious or fatal disease, such work necessitates operation in biosafety-level 3 (BSL-3) environments.
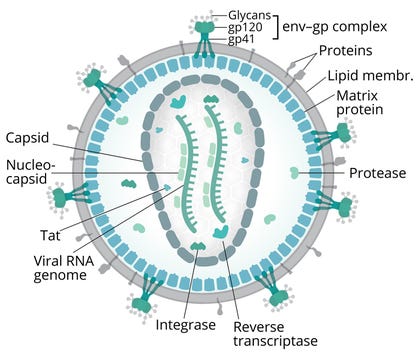
Figure 1: Diagram of human immunodeficiency virus (HIV); env = viral-envelope protein, gp = glycoprotein, Tat = transactivator of transcription protein
(adapted from Splettstoesser/Scistyle.com, 13)
Thus, for phase-1 clinical-trial manufacturing, Creo SG has partnered with Le Forestier’s company, Naobios, which is a good manufacturing practice (GMP)–certified contract development and manufacturing organization (CDMO) based in Saint-Herblain, France. Naobios specializes in preclinical and early clinical (through phase 2) bioprocess development and manufacturing for virus-based products, including viral vectors, viral vaccines, oncolytic viruses, and human viral-challenge agents (HVCAs). As the UNAIDS 2025 checkpoint approached, I spoke with Choi and Le Forestier to learn about the SAV001 candidate and its manufacturing process. Choi explained how Creo SG’s candidate could improve upon previous HIV-vaccine development approaches. Le Forestier described the relative complexity of BSL-3 environments compared with facilities with more typical biomanufacturing conditions. He also reflected on the state of contract manufacturing for virus-based products, including the need for BSL-3 capabilities in support of novel vaccines.
Improving Vaccine Efficacy
Several factors complicate HIV-vaccine development (8). Particularly onerous biological issues include the pathogen’s high rates of mutation and recombination during error-prone reverse-transcriptase–mediated replication processes. Those phenomena introduce significant variability into HIV env gp forms, which are the primary targets of neutralizing antibodies (nAbs). HIV also shields key glycoproteins with other glycans, improving chances for immune escape (Figure 1) (13).
HIV’s extensive genetic diversity would seem to hamper typical vaccine approaches based on specific antigens, as is the case with protein-subunit vaccines. Choi explained, “HIV-1’s high mutation rate and broad range of globally circulating viral strains suggest that a single-antigen immunization is unlikely to exert a therapeutic effect — and that an effective vaccine should incorporate multiple, conserved viral antigens.” The latter approach is technically possible, and some “mosaic” vaccines have entered preclinical and clinical development (8). Nevertheless, Choi observed, candidates using select viral components typically have not fared well during clinical studies: “Their efficacy in phase-2 and phase-3 clinical trials has been unimpressive.”
Compared with a subunit-vaccine approach, using whole but inactivated pathogens could have the advantage of “expressing virtually all viral proteins to the host immune system in their natural, mature conformations,” Choi continued. That principle has been proven sufficiently, and inactivated-pathogen vaccines have helped to inoculate global populations against a breadth of diseases, including polio, hepatitis A, Japanese encephalitis, and tick-borne encephalitis. That said, inactivation-based approaches “largely have been neglected for HIV-1 vaccines despite the ability of inactivated but intact, whole-virus vaccines to generate strong, predominantly antibody-mediated immune responses in vivo.”
Industry hesitance to pursue the approach stems not only from safety concerns, including potential for integration of proviral HIV DNA into host-cell genomes, but also from lackluster results in previous studies. Historically, candidates based on inactivated HIV have shown limited efficacy because the applied chemical and/or heat treatments destroyed virions’ key immune-stimulating molecules (8, 12). However, Choi explained, recent developments in inactivation methods “have greatly enhanced the utility of inactivated-virus immunogens.” For instance, chemical treatments now provide “elimination of virus infectivity to undetectable levels while maintaining the native protein conformation, including that of the important HIV-1 env gp120, which is the major target for nAb responses in vivo.” With current technologies, today’s drug developers can leverage a killed, whole–HIV-1 vaccine approach both safely and effectively.
Genetic modification can provide further assurance. Creo SG has engineered the SAV001 construct to generate noncytotoxic, avirulent progeny viruses that are capable of high-titer replication in a production cell line. Modifications include partial deletion of the nef gene (encoding for negative regulatory factor in primate lentiviruses) and replacement of genes for the env natural signal peptide (NS) with those for honeybee melittin signal peptide (MS). Choi explained that partial nef deletion “reduces the viral infectivity level by as much as 10-fold relative to the wild-type virus.” Replacing genes for NS likewise decreases infectivity, and that result “acts synergistically with nef deletion, exhibiting a >20-fold decrease in virus infectivity relative to wild-type controls.” Chemical and radiological inactivation of those minimally infective constructs ensures that vaccinated subjects will be safe from infection.
Results from a recent phase-1 clinical trial confirm the candidate’s safety and tolerability. The study evaluated intramuscular administration of a single SAV001 dose to men and women living with chronic HIV-1 infection who had been receiving combination ART (cART). Choi explained, “As a secondary measure, we also evaluated the vaccine-elicited humoral immune responses against the structural proteins of HIV-1, including nAb activity in the vaccinated individuals.” She added, “To our knowledge, this is the first human clinical trial with a killed, whole–HIV-1 vaccine.” SAV001 administration was safe and well tolerated in the studied cohort, with no life-threatening adverse events (AEs), serious adverse events (SAEs), or deaths.
Choi continued, “Our results suggest that the SAV001 vaccine will mimic natural infection through its native viral structure, especially the native form of the env gp, which is crucial for eliciting broadly neutralizing antibodies (bnAbs).” In another promising result, sera from trial participants who received vaccination with HIV-1 subtype B were able to neutralize subtypes A and D as well as B. “That is consistent with the notion that HIV-1 superinfection is a limited event,” she said, “particularly for patients who have been infected with HIV-1 for more than several months. Thus, HIV-1 vaccines based on one subtype may be able to protect against infections of other subtypes.” Creo SG will explore that possibility in phase-2 clinical trials involving HIV-negative participants.
Leveraging Specialized Capabilities
With clinical trials advancing, Creo SG needed to identify a contract-manufacturing partner that could support scale-up for a production process with rigorous biosafety requirements. Production of the SAV001 candidate involves cultivation and manipulation of live HIV, necessitating BSL-3 capabilities and rigorous means for ensuring safety and regulatory compliance — in addition to other qualities that program sponsors need from contract partners. “Creo SG had worked with a few other CDMOs for SAV001 GMP manufacturing and process development,” Choi reported, “but we often encountered inefficiencies or a lack of understanding of HIV characteristics at those companies.”
Serendipitously, Naobios had opened its CDMO business to the public in 2019, just a few years before Creo SG evaluated clinical-production prospects. During our conversation, Le Forestier highlighted the value of his company’s distinctive focus in viral-product manufacturing. Recognizing the presence of “much bigger industry players with expertise in adenoassociated virus (AAV) and lentivirus (LV)” for gene-therapy applications, company leadership decided that AAV and LV “would not be markets that we would enter unless they became advantageous for us.” Rather, the company established GMP capabilities for production of vaccines and challenge agents in BSL-2 and BSL-3 environments. HVCAs represent a relatively new product modality, he added. Specified doses of highly characterized pathogens can be administered to healthy volunteers (controlled human-infection models, CHIMs) to test vaccine safety and efficacy. Challenge agents gained traction during the COVID-19 pandemic as drug developers recognized their role in streamlining clinical studies. Public-health experts expect such products to remain highly valuable for testing vaccines against emerging pathogens of concern (14).
Choi and Le Forestier agreed that a major challenge facing drug developers such as Creo SG is that few CDMOs offer BSL-3 services. Contract manufacturers that work primarily with recombinant proteins and other conventional biologics generally will not require such facilities, Le Forestier said. Except when a strong business case can be made, establishing such capabilities would be both impractical and expensive for many contract organizations.
Le Forestier explained that BSL-3 facilities provide “an enhanced level of containment” compared with BSL-1 and BSL-2 environments. Air flow throughout a facility is a critical concern: “You need to enter a cleanroom through a personnel airlock with a specific design to ensure a pressure differential, with the cleanroom having negative pressure compared with the external environment. To prevent the spread of viral particles, you must use additional filters for air flowing out of the cleanroom. And you must leave the room through another separate personnel airlock.” Work with BSL-3 pathogens requires specialized decontamination procedures: “You need a double-door autoclave to decontaminate solid waste. Specific equipment is required for decontaminating liquid waste. And when you finish a manufacturing campaign, you must perform full decontamination of the production suite using an agent such as vaporized hydrogen peroxide.” Potential for broad contamination necessitates collaboration with local authorities, who must permit facilities and operations.
Facility considerations are just the tip of the iceberg. Technicians require extensive training to prepare for a number of potential contamination events. To that end, Le Forestier emphasized the need for detailed risk assessment: “We need to imagine many different scenarios — a fire breaking out or an operator fainting in a cleanroom. Then we perform training. For instance, we might perform trials to make sure that operators can escape a fire and work with our local fire department to alert them to the specificities of the contamination potential.”
Such work occurs in addition to training for specific virus-handling techniques, and different pathogens can require distinct protocols. Consider sudden acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and HIV-1, both of which Naobios has handled for customers. “SARS-CoV-2 is a respiratory virus, so when making challenge agents, we have needed to be extremely careful about generating sprays of small particles and so on,” Le Forestier said. “Those are less of a concern for HIV, but we definitely need to account for ways in which operators can hurt themselves — for instance, by having an accident that creates an open wound.” After thorough risk assessment, Naobios decided to mitigate such concerns by removing all sharp objects from the production suite. “We even considered different techniques for counting viruses and cells,” he added. “We decided to remove manual microscopes because they would require handling of glass slides. That is the level of specificity that we have needed during risk analysis. The whole system is fairly complex.”
Facilitating Manufacturing
SAV001’s production process raises some distinctive considerations in addition to perennial challenges associated with scale-up. Wild-type HIV grows slowly in infected cells. “Because of that,” Le Forestier explained, “we need to grow our production cells, then infect them with the gene-modified HIV and continue the culture.” Thus, Naobios developed a perfusion-mode bioreactor process to remove spent media and renew cells with fresh media. Subsequent purification involves familiar chromatography steps. To ensure that virions in a final product will be incapable of infecting vaccine-recipient cells, purified material undergoes chemical inactivation using 2,2'-dithiodipyridine (aldrithiol 2, AT-2), which covalently modifies essential zinc fingers in HIV nucleocapsid proteins (15). For extra assurance, treated material undergoes gamma irradiation.
Le Forestier credits his company’s successful management of such processes to his team’s significant experience and broad expertise. Although the team began operating as Naobios in 2019, its operations and the Nantes-area facility date back to 2005. “We are building on our expertise rather than starting from scratch,” he observed. That has enabled Naobios to handle, for instance, the complexity of perfusion culture of a BSL-3 pathogen relative to standard batch processing. Over time,
the company has worked with multiple viruses, product modalities, production approaches, and purification processes. Thus, it can offer customers platform-agnostic manufacturing: “Each time a customer approaches us, we can develop most everything for them.”
The company’s small size lends well to its versatile manufacturing model, Le Forestier observed. Although Naobios employs only about 40 people, their broad expertise enables them to take on highly specialized projects. “A much larger CDMO might prefer to use its own platform” to ensure standardization in operations, he explained, “and extremely large companies might be unable to stay responsive to some of their partners’ requests.” And despite its small size, Naobios can support complicated workflows by leveraging capabilities within the Clean Biologics network. For SAV001, Naobios performs AT-2 inactivation internally and works with a subcontractor for the gamma irradiation step. Then, sister company and biosafety-testing specialist Clean Cells performs assays to ensure that irradiated vaccine material contains no live virus. In-network capabilities streamline communication and decrease time to results for key assays, enabling project teams to make process adjustments quickly.
Choi agreed about the value of such an approach. She commended Naobios’s “highly efficient” workflows, “swift and clear” client communication, and possibilities for integrated services. Because of such offerings and close collaboration, “Naobios and Creo SG have succeeded in implementing process improvements for large-scale production.”
Cultivating a “Healthy Biotechnology Ecosystem”
Public-health authorities agree about the significant value of effective, low-cost HIV vaccines. Over time, cART will gain in efficacy while decreasing in cost, especially as generics proliferate (4). However, geopolitical and socioeconomic factors are likely to continue interrupting access to treatment, as suggested by the world’s uneven progress toward the 95–95–95 targets for 2025. Such considerations should give additional urgency to HIV-vaccine R&D. That includes the need for sustained innovation in manufacturing techniques and business models.
According to Le Forestier, the vaccine industry needs to cultivate a “healthy biotechnology ecosystem.” Obviously, CDMOs depend on drug developers for projects, but funding vacillates considerably in vaccine R&D. The SARS-CoV-2 pandemic exposed such financial vicissitudes. In many cases, significant flows of private investment quickly dried up once mRNA-based vaccines became available and demand for doses in wealthy nations tapered off. In Le Forestier’s experience, developers with public grants fared better than did companies that depended primarily on private funding, perhaps because the time required for public-funding approval and allocation gave developers money to operate sustainably over an extended period.
Shifts in vaccine-R&D investment have consequences, with contract manufacturers of viral products often experiencing surges and drops in requests for services. Le Forestier observed that CDMOs specializing in AAVs and LVs for gene-therapy applications were particularly susceptible to economic shifts at the beginning of the COVID-19 pandemic. Naobios offset changes in demand for specific vaccines with increased work in HVCAs, including SARS-CoV-2 products for CHIM studies. Ultimately, consistent funding streams and broad, flexible capabilities among manufacturers could help to maintain a healthy ecosystem for vaccines and other virus-based products.
The vaccine industry also would do well to establish further BSL-3 manufacturing capabilities for addressing the requirements of novel clinical candidates, such as SAV001. Le Forestier pointed out that several BSL-3 virus-production facilities operate in the United States, but often such facilities are affiliated with academic centers. Those laboratories are “accessible for early stage projects,” he explained, “but as soon as a project ‘graduates’ beyond early research, its developer needs to find an alternative facility to continue the work.” Considerable need remains for BSL-3–equipped contract manufacturers. Creo SG’s SAV001 represents an innovative combination of modern genetic engineering and classical pathogen-inactivation techniques, and Naobios’s specialized expertise is enabling scale-up for trials. Establishing further BSL-3 infrastructure for both clinical and commercial applications could help to bring vaccines to at-risk populations around the world.
References
1 Ending Inequalities and Getting on Track To End Aids by 2030. Joint United Nations Programme on HIV/AIDS: Geneva, Switzerland, 2021; https://www.unaids.org/sites/default/files/media_asset/2021-political-declaration_summary-10-targets_en.pdf.
2 Scott GY, Worku D. HIV Vaccination: Navigating the Path to a Transformative Breakthrough — A Review of Current Evidence. Health Sci. Rep. 7(9) 2024: e70089; https://doi.org/10.1002/hsr2.70089.
3 Payne D, et al. Trends in HIV Prevalence, Incidence, and Progress Towards the UNAIDS 95–95–95 Targets in Malawi Among Individuals Aged 15–64 Years: Population-Based HIV Impact Assessments, 2015–16 and 2020–21. The Lancet HIV 10(9) 2023: e597–e605; https://doi.org/10.1016/S2352-3018(23)00144-3.
4 Heath K, Levi J, Hill A. The Joint United Nations Programme on HIV/AIDS 95–95–95 Targets: Worldwide Clinical and Cost Benefits of Generic Manufacture. AIDS 35(s2) 2021: s197–S203; https://doi.org/10.1097/QAD.0000000000002983.
5 2024 Global AIDS Report — The Urgency of Now: AIDS at a Crossroads. Joint United Nations Programme on HIV/AIDS: Geneva, Switzerland, 2024; https://www.unaids.org/en/resources/documents/2024/global-aids-update-2024.
6 Kaur A, Vaccari M. Exploring HIV Vaccine Progress in the Pre-Clinical and Clinical Setting: From History to Future Prospects. Viruses 16(3) 2024: 368; https://doi.org/10.3390/v16030368.
7 HIV Vaccine Development. US National Institute of Allergy and Infectious Diseases: Bethesda, MD, 2019; https://www.niaid.nih.gov/diseases-conditions/hiv-vaccine-development.
8 Ng’uni T, Chasara C, Ndhlovu ZM. Major Scientific Hurdles in HIV Vaccine Development: Historical Perspective and Future Directions. Front. Immunol. Sec. Vacc. Mol. Ther. 11, 2020; https://doi.org/10.3389/fimmu.2020.590780.
9 Feinberg MB. Uhambo — Twists and Turns on the Journey to an Efficacious HIV-1 Vaccine. New Engl. J. Med. 384(12) 2021: 1157–1159; https://doi.org/10.1056/NEJMe2102358.
10 HIV Vaccine Candidate Does Not Sufficiently Protect Women Against HIV Infection (press release). US National Institutes of Health: Bethesda, MD, 31 August 2021; https://www.nih.gov/news-events/news-releases/hiv-vaccine-candidate-does-not-sufficiently-protect-women-against-hiv-infection.
11 Phase 3 Mosaic-Based Investigational HIV Vaccine Study Discontinued Following Disappointing Results of Planned Data Review (press release). HIV Vaccine Trials Network: Seattle, WA, 18 January 2023; https://www.hvtn.org/news/news-releases/2023/01/phase-3-mosaic-based-investigational-hiv-vaccine-study-discontinued-following-disappointing-results-planned-data-review.html.
12 Harris JE. The Repeated Setbacks of HIV Vaccine Development Laid the Groundwork for SARS-CoV-2 Vaccines. Health Policy Technol. 11(2) 2022: 100619; https://doi.org/10.1016/j.hlpt.2022.100619.
13 Splettstoesser T. Diagram of the HIV Virion (image). Wikimedia Commons, 26 June 2014; https://en.wikipedia.org/wiki/File:HI-virion-structure_en.svg.
14 Balasingam S, et al. Human Infection Studies: Key Considerations for Challenge Agent Development and Production. Wellcome Open Res. 7, 2022: 140; https://doi.org/10.12688/wellcomeopenres.17869.1.
15 Rossio JL, et al. Inactivation of Human Immunodeficiency Virus Type 1 Infectivity with Preservation of Conformational and Functional Integrity of Virion Surface Proteins. J. Virol. 72(10) 1998: 7992–8001; https://doi.org/10.1128/jvi.72.10.7992-8001.1998.
Brian Gazaille, PhD, is managing editor of BioProcess International, part of Informa Connect Life Sciences;
[email protected]. Eunsil Choi, DVM, is director
of drug development at Sumagen Canada Inc./Creo SG Co., Ltd. And Eric Le Forestier is general manager of Naobios.
You May Also Like




