A team of international scientists has demonstrated that therapeutic inhibition of a transcriptional regulator BRD2, required for the endogenous expression of angiotensin-converting enzyme 2 (ACE2), can potentially suppress severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. The study is currently available on the bioRxiv* preprint server.
Background
The ongoing coronavirus disease 2019 (COVID-19) pandemic caused by SARS-CoV-2 is imposing much socioeconomic, health, and environmental challenges to the global population. As of January 2021, there have been 99.17 million confirmed COVID-19 cases, including over 2.1 million deaths, reported to the World Health Organization. To prevent the rapid spread of SARS-CoV-2, the entire scientific community is scrambling to understand the molecular mechanisms associated with virus-host interaction.
It is now well established that the interaction between SARS-CoV-2 spike protein and host ACE2 is a prerequisite for viral entry into host cells. Therefore, cellular components perturbing this interaction could serve as vital therapeutic targets to prevent SARS-CoV-2 infection.
In the current study, the scientists have conducted a CRISPR interference (CRISPRi)-based screening for cellular components that can modify the spike – ACE2 interaction. In particular, they have looked for components that can affect the endogenous expression of ACE2.
Study design
To perform the CRISPRi screening, the scientists have selected a lung epithelial cancer cell line that endogenously expresses ACE2. The highest response has been observed for genes that are significantly associated with ACE2 expression. In the next step, they have silenced these genes and analyzed their impact on ACE2 expression. Moreover, they have analyzed whether these genes can potentially affect SARS-CoV-2 infection.
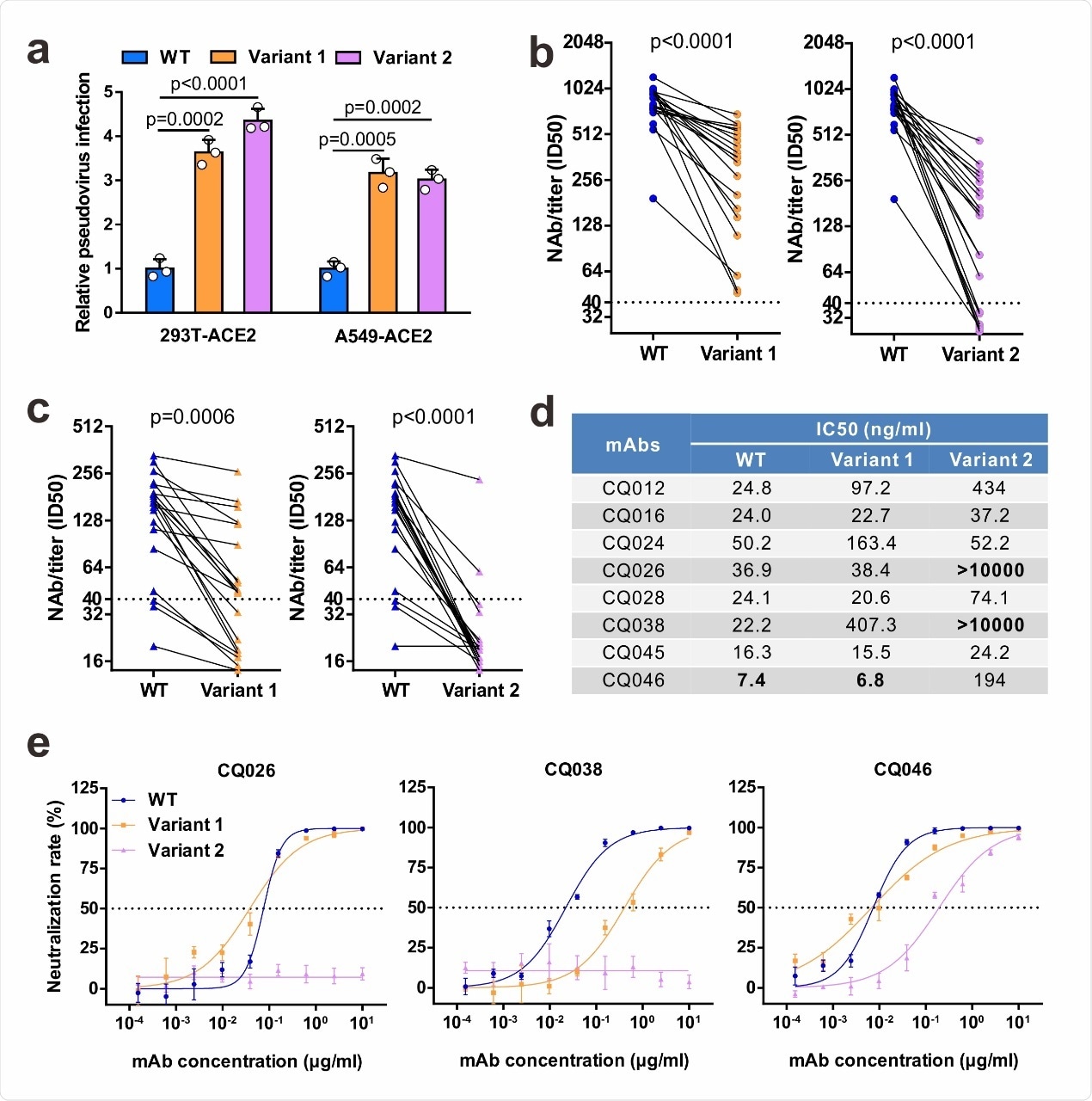
Neutralizing activities of convalescent sera and monoclonal antibodies against SARS-CoV-2 variants. a Infectivity of WT and variant pseudovirus conducted in 293T-ACE2 and A549-ACE2 cells. Cells were inoculated with equivalent doses of each pseudotyped virus. WT, wild-type Spike (GenBank: 213 QHD43416) pesudotyped virus; Variant 1, N501Y.V1 mutant Spike pesudotyped virus (containing H60/V70 deletion, Y144 deletion, N501Y, 215 A570D, D614G, P681H, T716I, S982A, D1118H); Variant 2, N501Y.V2 mutant Spike pesudotyped virus (containing K417N, E484K, N501Y, D614G). b-c Neutralization of WT and variant pseudoviruses by convalescent sera. Pseudovirus-based neutralizing assay were performed to detect neutralizing antibody (NAb) titers against SARS-CoV-2. The thresholds of detection were 1:40 of ID50. Twenty sera (indicated by circles) were drawn 5 to 33 days post-symptom onset (b); 20 sera (indicated by triangles) were drawn ~ 8 months post-symptom onset (c). d-e The half-maximal inhibitory concentrations (IC50) for tested monoclonal antibodies (mAbs) against pseudoviruses (d) and representative neutralization curves (e). Statistical significance was determined by One-way ANOVA.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Important observations
Using CRISPRi-based genetic screening, the scientists specifically checked for cellular components that regulate the binding of spike receptor-binding domain (spike-RBD) with ACE2. Based on the most substantial hits observed in the screening, they have finally selected five genes; of which, silencing of ACE2 and BRD2 genes reduces the spike-RBD binding, and silencing of CDC7, COMP, and TRRAP genes increase the spike-RBD binding.
Interestingly, they have observed that the genes associated with a lower level of a spike-RBD binding act at the transcriptional level to reduce the expression of ACE2. Similarly, the genes associated with higher spike-RBD binding has been found to increase the ACE2 transcript levels.
Regarding the association between target gene silencing and SARS-CoV-2 infection, they have observed that silencing of BRD2 leads to complete inhibition of viral replication inside host cells even after 72 hours of infection. Moreover, the intensity of the effect is equivalent to that observed in ACE2 silencing.
Given the strong impact of BRD2 on vital infection, they have investigated the therapeutic potential of BRD2 in treating COVID-19. Specifically, they have investigated the effects of several small molecule inhibitors of BRD2, which are currently under clinical trials, on ACE2 expression and spike-RBD binding. The findings have revealed that BRD2 inhibitors significantly reduce the expression of ACE2 mRNA in human lung epithelial cells and cardiomyocytes without causing any cytotoxicity. Notably, the treatment of SARS-CoV-2-infected cells with these inhibitors leads to a 100-fold reduction in viral replication.
To determine the mode of action of BRD2, they have conducted a series of experiments, which have revealed that BRD2 directly regulates the transcription of ACE2. Interestingly, they have observed that in addition to reducing ACE2 expression, the inhibition of BRD2 leads to downregulation of genes induced by SARS-CoV-2 infection, such as type I interferon response genes.
Study significance
The study identifies BRD2 as a potent regulator of ACE2 expression and spike-RBD binding. Given the study findings, BRD2 can be a potential therapeutic target to treat COVID-19 patients. Based on previous study observations, the scientists suggest that the interaction between SARS-CoV-2 envelop protein and BRD2 might have evolved to regulate the expression of ACE2 during infection.
Besides inhibiting the viral entry through ACE2 downregulation, BRD2 inhibition can prevent the SARS-CoV-2-induced aberrant immune responses by downregulating type I interferon response genes.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Article Revisions
- Apr 4 2023 - The preprint preliminary research paper that this article was based upon was accepted for publication in a peer-reviewed Scientific Journal. This article was edited accordingly to include a link to the final peer-reviewed paper, now shown in the sources section.